Solid-State Batteries: Benefits and Drawbacks

Batteries are one of the cornerstones of the technological evolution of the past decades. They make every electricity dependent technology useable off grid. From using a simple flashlight, to calling family in an electric vehicle in the middle of nowhere, batteries provide endless opportunities that are taken for granted in our current society. A key restriction on this increased practicality is the need for charging, a bottleneck that is most discussed in the context of electric vehicles, inspiring a search for technologies that allow better range.
The importance of batteries will only increase, given the current climate transition; one of the main challenges of renewable energy is the energy storage capacity. Therefore, one of the more interesting developments in battery-technology would be increased lifetime and storage, which comes down to the attainable energy density; same size batteries that can store more energy than the ones we have currently have.
A promising emerging technology, the solid-state battery, has a lot of potential to offer a solution, and may even replace lithium-ion batteries in the long run. Before it does, some key bottlenecks need to be addressed.
The difference between lithium-ion batteries and solid-state batteries
Lithium-ion batteries are the current battery of choice for commercial applications. It is a proven technology that is economically competitive and thus easily scalable for mass-use. The major drawback of these batteries are its modest energy density (around 250 Wh/kg) and safety concerns regarding thermal runaway, causing the battery to catch fire. Thermal runaways are rare in everyday use technologies but should be avoided if possible. Solid-state lithium-metal batteries could provide answers to these problems. With an improved safety and higher energy density of around 400 Wh/kg, they have the ability to not only improve current technology (lifetime, charge time, etc.), but provide new opportunities like electric flight. To understand where these advantages come from, we need to understand the difference in working mechanisms.
Lithium-ion batteries have three key components: a negatively charged anode, a positively charged cathode and a separator in between. The anode and cathode are porous, allowing for liquid electrolyte to move positively charged lithium between the two electrodes. This movement of charge causes free electrons in the anode to move to the cathode, creating a current. This current provides the electricity that is used to operate a cellphone or an EV. An empty battery is charged by reversing this process.
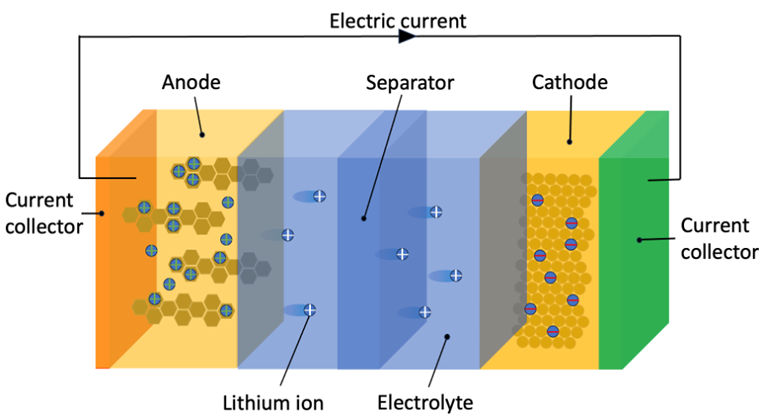
Discharging lithium-ion battery. (Figure: Econopolis Strategy)
In solid state batteries the liquid electrolyte is replaced by a solid electrolyte, this makes it an All-solid-state battery (ASSB). As the liquid electrolyte poses the most danger in terms of flammability, the solid-state battery is much safer. Solid electrolytes also are compatible with better performing anode materials such as silicon or lithium metal. Lithium metal has about 10 times the specific capacity of the graphite that is typically used in lithium-ion batteries, increasing the energy density. The three most researched solid-state batteries use electrolyte materials such as sulfide, oxide and polymers. These all have different metrics in terms of safety, production difficulty and performance.
Characteristics of different electrolyte materials in solid-state batteries
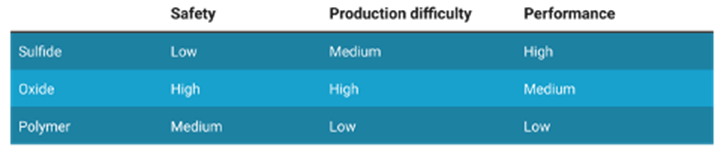
(Table: Econopolis Strategy)
Drawbacks of solid-state batteries
All types of batteries lose a significant amount of their theoretical potential due to practical limitations, but a solid-state lithium-metal battery still outperforms lithium-ion batteries in terms of safety and energy density. One of the major drawbacks, however, are the degradation mechanisms in the solid-state type batteries. The solid electrolyte does not perfectly block lithium dendrites from forming when charging. This causes a short circuit if it reaches the cathode. Discharging the battery on the other hand, can lead to interfacial delamination, causing spots on the anode to lose contact with the electrolyte. Furthermore, combined with phenomena like lithium creep and dead lithium, these batteries need replacement after extensive charge and recharge sequences.
Forming of dendrites over lifetime of solid-state batteries
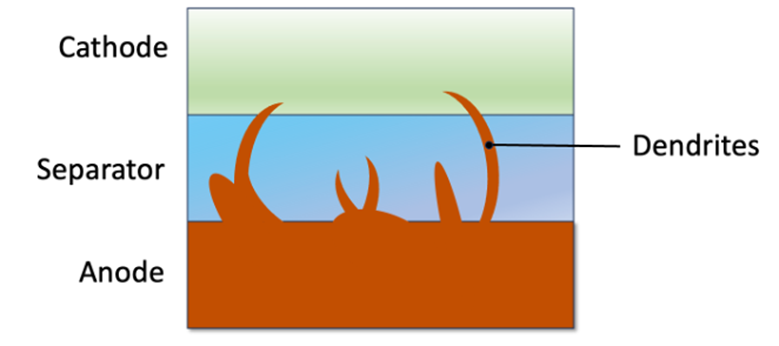
(Figure: Econopolis Strategy)
Other important challenges are cost and usability. The handling and manufacturing of solid-state batteries are more complex, which is reflected in the cost. This also prohibits the mass production and integration of these types of batteries in everyday use. Other restrictions are caused due to useability. Solid-state batteries have had poor performances while operating at low temperatures. Stabilizing them to be useful at room temperatures is not always a given. Furthermore, pressure considerations make them more fragile. All these issues can be fixed but will come with a cost in terms of lowering the energy density. Nevertheless, researchers are hopeful solid-state batteries will find their applications in pacemakers, wearables, electric vehicles and space equipment.
Solid-state batteries and climate transition
Due to higher energy density in solid-state batteries, the storage capacity of renewable energy could reach all-time highs, allowing for less waste in the energy supply chain. The reduced weight of the battery causes reduced material use and can lower the wear for electric vehicles specifically. The Brussel based campaign group ‘Transport and Environment’ reported a 39% decrease in carbon footprint of electric vehicles by converting to solid-state. These types of batteries also avoid the use of dangerous and toxic materials, reducing environmental risks.
Another possible advantage could be a reduced use of critical raw materials. The lithium use is projected to go up while the cobalt use should significantly reduce. It is still unclear to what extend cobalt is replaced by other critical materials like nickel or manganese. As the solid-state batteries are still new technologies and companies protect their intellectual property, it is too early to say if solid-state would reduce critical raw materials demand.
Solid-state batteries are an exciting new technology that can improve the existing battery paradigm and open gateways towards new technology developments. They still suffer, however, of practical implementation difficulties. Just like nuclear fusion, the theoretical potential is huge, but it needs to be practically attainable. Hopefully, with steady research progression, these batteries can help shape our renewable future.